- Atomic Number 33
- Atomic Number And Mass Number Worksheet
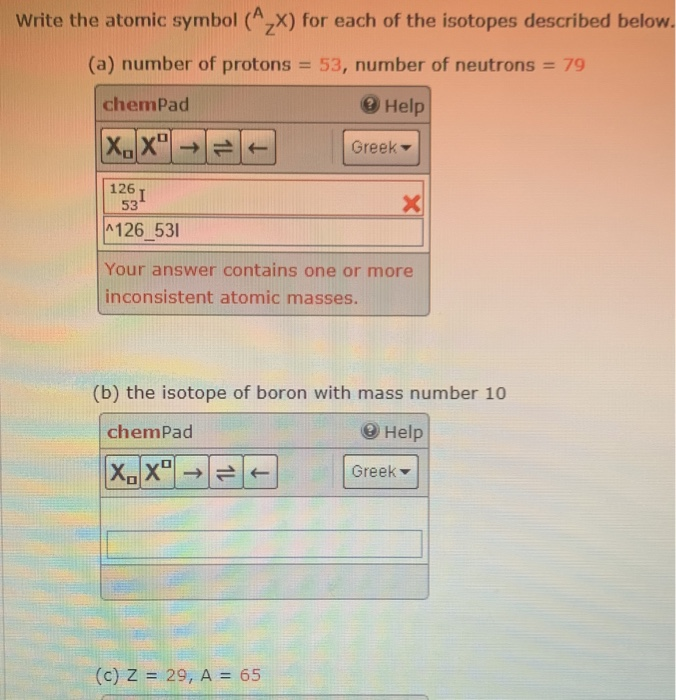
- Atomic Number 53
- Atomic Number 53 On Periodic Table
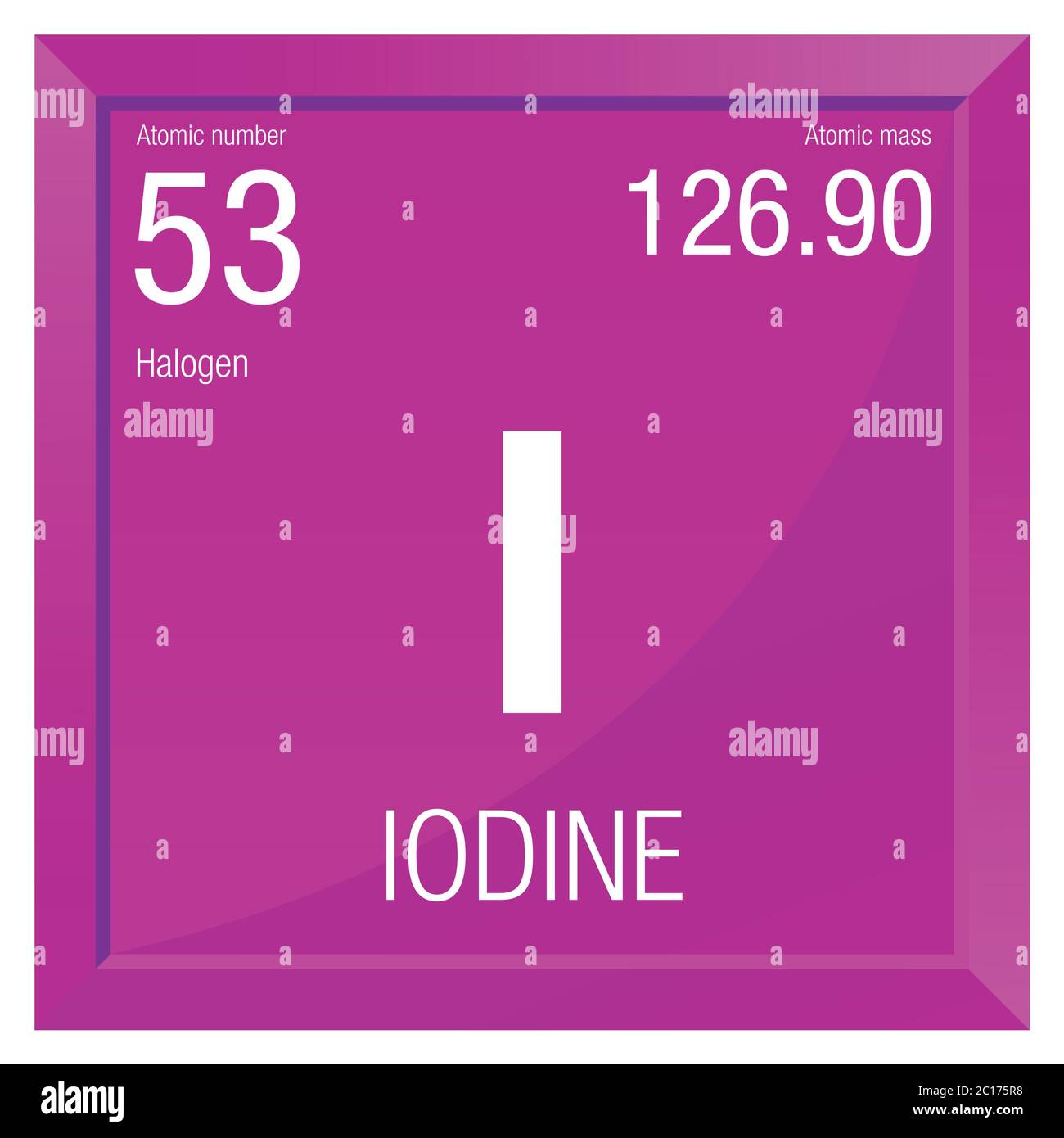
Definition of atomic number 53 in the Definitions.net dictionary. Meaning of atomic number 53. What does atomic number 53 mean? Information and translations of atomic number 53 in the most comprehensive dictionary definitions resource on the web. Definitions of atomic number 53 noun a nonmetallic element belonging to the halogens; used especially in medicine and photography and in dyes; occurs naturally only in combination in small quantities (as in sea water or rocks). Iodine (I) is a purple grey solid non metal. It has the atomic number 53 in the periodic table. It is located in Group 17, the Halogens. It has the symbol I. Iodine was discovered in 1811 by Bernard Courtois when he was trying to extract potassium chloride from seaweed.
We remember from our school chemistry course that every element has its own specific atomic number. It is the same as the number of protons that the atom of each element has, so sometimes atomic number is called proton number. It is always the whole number and it ranges from 1 to 118, according to the number of the element in the Periodic Table. This number can be really important and something essential to know, in relation to a certain chemical element which is the issue of our interest at the moment.
- Iodine is a chemical element with the symbol I and atomic number 53. The heaviest of the stable halogens, it exists as a lustrous, purple-black non-metallic solid at standard conditions that melts to form a deep violet liquid at 114 degrees Celsius, and boils to a violet gas at 184 degrees Celsius. However, it readily sublimes with gentle heat, resulting in a widespread misconception even.
- The atomic mass is useful in chemistry when it is paired with the mole concept: the atomic mass of an element, measured in amu, is the same as the mass in grams of one mole of an element. Thus, since the atomic mass of iron is 55.847 amu, one mole of iron atoms would weigh 55.847 grams.
Why is this so? Why is the atomic number so important? First of all, it is the number that makes elements different from one another as it shows the number of protons in their nuclei. Also, knowing the atomic number of an element can give us an idea about the position of the element in the Periodic Table. Atomic number of an element never changes: for example, the atomic number of oxygen is always 8, and the atomic number of Chlorine is always 18. The atomic number is marked with the symbol Z, taken from a German word zahl (or atomzahl, which is 'atomic number' in German).

This website is created for those who need to know the atomic number of a central chemical element. By using our website, you can do it in just one click and receive short and correct information on this matter. There is also some extra summary on every each chemical element which can be found at our website, including the atomic weight of each element, as well as physical and chemical properties of every element and its importance. Use this website at any time when you need to get fast and precise information about atomic or proton number of chemical elements.
List of chemical elements in periodic table with atomic number, chemical symbol and atomic weight. You can sort the elements by clicking on the table headers. Please click on the element name for complete list of element properties.
| Atomic Number | Chemical Symbol | Element Name | Atomic Weight (u) |
|---|---|---|---|
| 1 | H | Hydrogen | 1.008 |
| 2 | He | Helium | 4.003 |
| 3 | Li | Lithium | 6.94 |
| 4 | Be | Beryllium | 9.012 |
| 5 | B | Boron | 10.81 |
| 6 | C | Carbon | 12.011 |
| 7 | N | Nitrogen | 14.007 |
| 8 | O | Oxygen | 15.999 |
| 9 | F | Fluorine | 18.998 |
| 10 | Ne | Neon | 20.18 |
| 11 | Na | Sodium | 22.99 |
| 12 | Mg | Magnesium | 24.305 |
| 13 | Al | Aluminium | 26.982 |
| 14 | Si | Silicon | 28.085 |
| 15 | P | Phosphorus | 30.974 |
| 16 | S | Sulfur | 32.06 |
| 17 | Cl | Chlorine | 35.45 |
| 18 | Ar | Argon | 39.948 |
| 19 | K | Potassium | 39.098 |
| 20 | Ca | Calcium | 40.078 |
| 21 | Sc | Scandium | 44.956 |
| 22 | Ti | Titanium | 47.867 |
| 23 | V | Vanadium | 50.942 |
| 24 | Cr | Chromium | 51.996 |
| 25 | Mn | Manganese | 54.938 |
| 26 | Fe | Iron | 55.845 |
| 27 | Co | Cobalt | 58.933 |
| 28 | Ni | Nickel | 58.693 |
| 29 | Cu | Copper | 63.546 |
| 30 | Zn | Zinc | 65.38 |
| 31 | Ga | Gallium | 69.723 |
| 32 | Ge | Germanium | 72.63 |
| 33 | As | Arsenic | 74.922 |
| 34 | Se | Selenium | 78.971 |
| 35 | Br | Bromine | 79.904 |
| 36 | Kr | Krypton | 83.798 |
| 37 | Rb | Rubidium | 85.468 |
| 38 | Sr | Strontium | 87.62 |
| 39 | Y | Yttrium | 88.906 |
| 40 | Zr | Zirconium | 91.224 |
| 41 | Nb | Niobium | 92.906 |
| 42 | Mo | Molybdenum | 95.95 |
| 43 | Tc | Technetium | 98 |
| 44 | Ru | Ruthenium | 101.07 |
| 45 | Rh | Rhodium | 102.906 |
| 46 | Pd | Palladium | 106.42 |
| 47 | Ag | Silver | 107.868 |
| 48 | Cd | Cadmium | 112.414 |
| 49 | In | Indium | 114.818 |
| 50 | Sn | Tin | 118.71 |
| 51 | Sb | Antimony | 121.76 |
| 52 | Te | Tellurium | 127.6 |
| 53 | I | Iodine | 126.904 |
| 54 | Xe | Xenon | 131.293 |
| 55 | Cs | Caesium | 132.905 |
| 56 | Ba | Barium | 137.327 |
| 57 | La | Lanthanum | 138.905 |
| 58 | Ce | Cerium | 140.116 |
| 59 | Pr | Praseodymium | 140.908 |
| 60 | Nd | Neodymium | 144.242 |
| 61 | Pm | Promethium | 145 |
| 62 | Sm | Samarium | 150.36 |
| 63 | Eu | Europium | 151.964 |
| 64 | Gd | Gadolinium | 157.25 |
| 65 | Tb | Terbium | 158.925 |
| 66 | Dy | Dysprosium | 162.5 |
| 67 | Ho | Holmium | 164.93 |
| 68 | Er | Erbium | 167.259 |
| 69 | Tm | Thulium | 168.934 |
| 70 | Yb | Ytterbium | 173.045 |
| 71 | Lu | Lutetium | 174.967 |
| 72 | Hf | Hafnium | 178.49 |
| 73 | Ta | Tantalum | 180.948 |
| 74 | W | Tungsten | 183.84 |
| 75 | Re | Rhenium | 186.207 |
| 76 | Os | Osmium | 190.23 |
| 77 | Ir | Iridium | 192.217 |
| 78 | Pt | Platinum | 195.084 |
| 79 | Au | Gold | 196.967 |
| 80 | Hg | Mercury | 200.592 |
| 81 | Tl | Thallium | 204.38 |
| 82 | Pb | Lead | 207.2 |
| 83 | Bi | Bismuth | 208.98 |
| 84 | Po | Polonium | 209 |
| 85 | At | Astatine | 210 |
| 86 | Rn | Radon | 222 |
| 87 | Fr | Francium | 223 |
| 88 | Ra | Radium | 226 |
| 89 | Ac | Actinium | 227 |
| 90 | Th | Thorium | 232.038 |
| 91 | Pa | Protactinium | 231.036 |
| 92 | U | Uranium | 238.029 |
| 93 | Np | Neptunium | 237 |
| 94 | Pu | Plutonium | 244 |
| 95 | Am | Americium | 243 |
| 96 | Cm | Curium | 247 |
| 97 | Bk | Berkelium | 247 |
| 98 | Cf | Californium | 251 |
| 99 | Es | Einsteinium | 252 |
| 100 | Fm | Fermium | 257 |
| 101 | Md | Mendelevium | 258 |
| 102 | No | Nobelium | 259 |
| 103 | Lr | Lawrencium | 266 |
| 104 | Rf | Rutherfordium | 267 |
| 105 | Db | Dubnium | 268 |
| 106 | Sg | Seaborgium | 269 |
| 107 | Bh | Bohrium | 270 |
| 108 | Hs | Hassium | 277 |
| 109 | Mt | Meitnerium | 278 |
| 110 | Ds | Darmstadtium | 281 |
| 111 | Rg | Roentgenium | 282 |
| 112 | Cn | Copernicium | 285 |
| 113 | Nh | Nihonium | 286 |
| 114 | Fl | Flerovium | 289 |
| 115 | Mc | Moscovium | 290 |
| 116 | Lv | Livermorium | 293 |
| 117 | Ts | Tennessine | 294 |
| 118 | Og | Oganesson | 294 |
Atomic Number 33
Atomic Number And Mass Number Worksheet
Lists of Elements in Periodic Table
You can also list the elements in various ordered properties with printable tables below.
Atomic Number 53
Lists of Elements by Group Number in Periodic Table
Atomic Number 53 On Periodic Table
» Group 1» Group 2» Group 3» Group 4» Group 5» Group 6» Group 7» Group 8» Group 9» Group 10» Group 11» Group 12» Group 13» Group 14» Group 15» Group 16» Group 17» Group 18