What does Valence mean in chemistry?
Do you know how Compounds are formed? From the Combination of Atoms right? But how and why do different Atoms combine? One of the crucial factors for this is. Key Differences – Valency vs Valence Electrons Valency electrons and valence electrons are inter-related terms, and the key difference between valency and valence electrons is best explained in their definitions; valence electrons are the electrons in the outermost shell of an element whereas valency electrons are the number of electrons that should be accepted or removed to attain the.
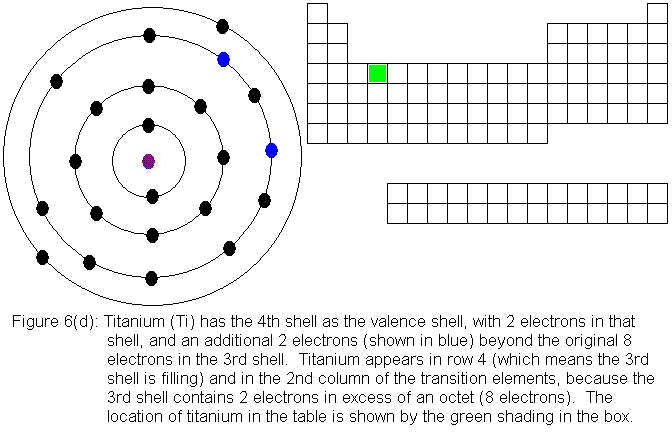
1 Answer
Valence is a term that deals with the electrons that are most typically involved in the bonding characteristics of an atom. These electrons are found in the s and p orbitals of the highest energy level of the electron configuration for the element.
The valence shell can hold 2 electrons in the s orbital representing the first two columns of the periodic table and 6 electrons in the p orbital found in columns 13 - 18 of the periodic table.

Some examples would include:
Mg
Se
Valence Chemistry Related People
Each element in the periodic table will have the same number of valence electrons as the elements in the same column. All Alkai Metals (Li, Na, K, Rb) have 1 valence electron. All Halogens (F.Cl, Br, I) have 7 valence electrons. All of the Noble Gases (Ne, Ar, Kr Xe) will have 8 valence electrons and fulfill the
Atoms try to gain or lose electrons to fulfill the
I HOPE THIS WAS HELPFUL.
SMARTERTEACHER
Valence Chemistry Examples
What Is Valence Chemistry
Related questions
